
 6
6Polyribosomes Are Molecular 3D Nanoprinters That Orchestrate the Assembly of Vault Particles
In a time in which efficient 3D manufacturing is predicted to have a revolutionary effect on mankind, nature unveils that it has already been using this technique for millions of years. Vaults are very large ribonucleoprotein particles found widely in eukaryotes. Our discovery of the unique assembly mechanism of the vault particle reveals an unforeseen function of the polyribosome as a very sophisticated cellular 3D nanoprinter. 4
Three-dimensional printers fabricate a gamut of products such as medical devices, toys, and even specialty chocolates. Now a new study suggests that eukaryotic cells evolved a 3-D nanoprinter millions of years ago, in the form of polyribosomes. These clusters of ribosomes strung along a single messenger RNA appear to be responsible for the intricate 3-D assembly of a mysterious large, barrel-shaped protein complex called the vault particle 5
“I had never in my life seen anything like these rolls,” Mrazek says. “Normally, when you get misassembled proteins you see ugly tangles, but these were so symmetric.” Mrazek concluded that the rolls must reflect structural intermediates during the vault assembly process. Ribosomes moving along an mRNA normally synthesize individual protein strands, which then come off the ribosome and fold into their 3-D shapes. On the basis of the proposed helical geometry of the polyribosome, Mrazek hypothesized that as an individual MVP molecule gets translated by a ribosome in the cluster, it could form a dimer with the MVP synthesized by the ribosome next to it. As they’re formed, adjacent dimers could then arrange side by side to form the vault particle. The vaults take shape bit by bit as the dimers are completed and come off the polyribosome, much like a 3-D printer might build up the layers of a plastic object. The sixth mutation in the MVP somehow disrupts the pinching off of the vault particle after the 39th dimer assembles, resulting in the long, rolled-up vaults.
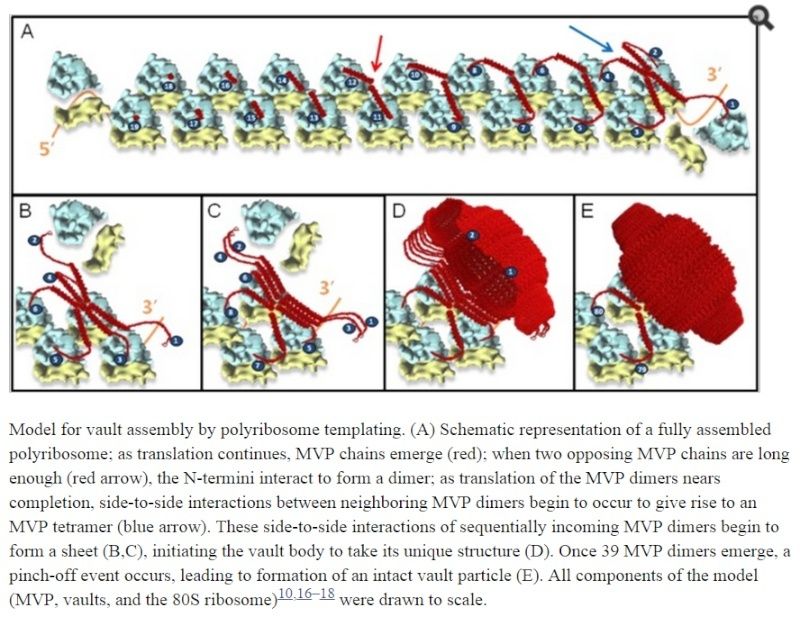
Ribosomes are molecular machines that function in polyribosome complexes to translate genetic information, guide the synthesis of polypeptides, and modulate the folding of nascent proteins. Here, we report a surprising function for polyribosomes as a result of a systematic examination of the assembly of a large ribonucleoprotein complex, the vault particle. Structural and functional evidence points to a model of vault assembly whereby the polyribosome acts like a 3D nanoprinter to direct the ordered translation and assembly of the multi-subunit vault homopolymer, a process which we refer to as polyribosome templating. Structure-based mutagenesis and cell-free in vitro expression studies further demonstrated the critical importance of the polyribosome in vault assembly. Polyribosome templating prevents chaos by ensuring efficiency and order in the production of large homopolymeric protein structures in the crowded cellular environment and might explain the origin of many polyribosome-associated molecular assemblies inside the cell.
Polyribosomes May Act As 3-D Nanoprinters To Fabricate Vault Particles

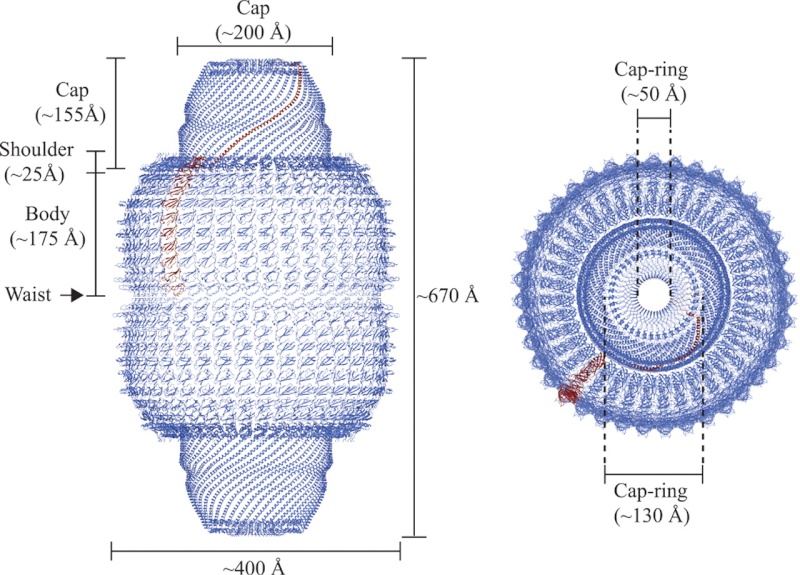
Vaults are large barrel-shaped ribonucleoprotein particles that are highly conserved in a wide variety of eukaryotes (1). Although several functions have been proposed for vaults since their discovery in 1986 (2–10), including roles in multidrug resistance, cell signaling, and innate immunity, their cellular function remains unclear. Most vault particles are present in the cytoplasm, but a few of them localize to the nucleus 1
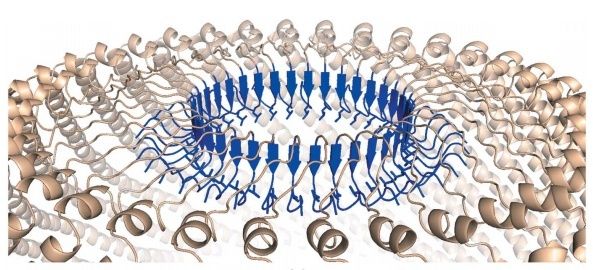
The proposal that vaults could act as a cellular transporter implies that these particles must present a highly regulated opening mechanism in order to efficiently incorporate and deliver the specific vault cargo. Even so, both the precise regulation of the opening mechanism and the structural characteristics that can allow such surprising dynamics still have to be clearly defined 2
A 2014 paper found “a surprising function for polyribosomes as a result of a systematic examination of the assembly of a large ribonucleoprotein complex, the vault particle”. Beyond merely orienting ribosomes in crowded conditions to avoid aggregation of freshly produced peptides, polyribosomes act “like a 3D nanoprinter” spinning out the many copies needed for this homopolymer, which they called ‘polyribosome templating’. That is, the interactions are not being minimised for fear of dangerous aggregation, but controlled to orchestrate favourable interactions to produce vaults. 3
Three-dimensional printers fabricate a gamut of products such as medical devices, toys, and even specialty chocolates. Now a new study suggests that eukaryotic cells evolved a 3-D nanoprinter millions of years ago, in the form of polyribosomes. These clusters of ribosomes strung along a single messenger RNA appear to be responsible for the intricate 3-D assembly of a mysterious large, barrel-shaped protein complex called the vault particle 5
“I had never in my life seen anything like these rolls,” Mrazek says. “Normally, when you get misassembled proteins you see ugly tangles, but these were so symmetric.” Mrazek concluded that the rolls must reflect structural intermediates during the vault assembly process. Ribosomes moving along an mRNA normally synthesize individual protein strands, which then come off the ribosome and fold into their 3-D shapes. On the basis of the proposed helical geometry of the polyribosome, Mrazek hypothesized that as an individual MVP molecule gets translated by a ribosome in the cluster, it could form a dimer with the MVP synthesized by the ribosome next to it. As they’re formed, adjacent dimers could then arrange side by side to form the vault particle. The vaults take shape bit by bit as the dimers are completed and come off the polyribosome, much like a 3-D printer might build up the layers of a plastic object. The sixth mutation in the MVP somehow disrupts the pinching off of the vault particle after the 39th dimer assembles, resulting in the long, rolled-up vaults.

Ribosomes are molecular machines that function in polyribosome complexes to translate genetic information, guide the synthesis of polypeptides, and modulate the folding of nascent proteins. Here, we report a surprising function for polyribosomes as a result of a systematic examination of the assembly of a large ribonucleoprotein complex, the vault particle. Structural and functional evidence points to a model of vault assembly whereby the polyribosome acts like a 3D nanoprinter to direct the ordered translation and assembly of the multi-subunit vault homopolymer, a process which we refer to as polyribosome templating. Structure-based mutagenesis and cell-free in vitro expression studies further demonstrated the critical importance of the polyribosome in vault assembly. Polyribosome templating prevents chaos by ensuring efficiency and order in the production of large homopolymeric protein structures in the crowded cellular environment and might explain the origin of many polyribosome-associated molecular assemblies inside the cell.

Polyribosomes May Act As 3-D Nanoprinters To Fabricate Vault Particles

Vaults are large barrel-shaped ribonucleoprotein particles that are highly conserved in a wide variety of eukaryotes (1). Although several functions have been proposed for vaults since their discovery in 1986 (2–10), including roles in multidrug resistance, cell signaling, and innate immunity, their cellular function remains unclear. Most vault particles are present in the cytoplasm, but a few of them localize to the nucleus 1
The proposal that vaults could act as a cellular transporter implies that these particles must present a highly regulated opening mechanism in order to efficiently incorporate and deliver the specific vault cargo. Even so, both the precise regulation of the opening mechanism and the structural characteristics that can allow such surprising dynamics still have to be clearly defined 2

A 2014 paper found “a surprising function for polyribosomes as a result of a systematic examination of the assembly of a large ribonucleoprotein complex, the vault particle”. Beyond merely orienting ribosomes in crowded conditions to avoid aggregation of freshly produced peptides, polyribosomes act “like a 3D nanoprinter” spinning out the many copies needed for this homopolymer, which they called ‘polyribosome templating’. That is, the interactions are not being minimised for fear of dangerous aggregation, but controlled to orchestrate favourable interactions to produce vaults. 3

So how are Vaults and their " made of " best explained ? Design, or natural mechanisms ?
1) http://www.sciencemag.org/content/323/5912/384.full
2) http://digital.csic.es/bitstream/10261/88208/1/New%20features.pdf
3) http://biochemistri.es/vault-particles
4) http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4245718/
5) http://cen.acs.org/articles/92/web/2014/11/Polyribosomes-Act-3-D-Nanoprinters.html
6) http://bioinformatics.org/firstglance/fgij//fg.htm?mol=2ZUO
Nenhum comentário:
Postar um comentário